Glaucoma is the second leading cause of blindness in the world. The current option available for the treatment glaucoma is to lower the intraocular pressure which is the only modifiable risk factor. Despite of continuous usage of glaucoma medications, there is still some proportion of the glaucoma patients progress to severe form of glaucoma and lose their vision. Therefore, there is always a constant search for the discovery of newer/better drugs for glaucoma. Our lab is mainly working on identifying newer therapeutic targets by looking at the various molecular mechanism (s) associated with the pathogenesis of glaucoma especially primary open angle glaucoma (POAG) and steroid-induced ocular hypertension (OHT)/glaucoma.
The research projects are supported by various Indian funding agencies such as SERB, ICMR, DBT as well as The Wellcome Trust/DBT India Alliance (India Alliance).
Development of Rho kinase inhibitor (s) for glaucoma
Our main research focus is to understand the role of RhoA /ROCK signaling in the pathogenesis of glaucoma and to evaluate the Rho kinase inhibitors (RKI) as a new class of IOP lowering drugs for its management. An unique ex vivo model system – human anterior segment perfusion culture (HOCAS) was established for this purpose using human donor eyes in collaboration with Dr. Kaufman, University of Wisconsin, Madison, USA.
Rho kinase is a small GTPase involved in the regulation of many cellular processes. In trabecular meshwork, rho kinase is involved in the synthesis of extracellular matrix components and permeability of Schlemm’s canal endothelial cells. The involvement of RhoA/ROCK signaling in glaucoma pathogenesis is well established and led to the discovery of FDA approved rho kinase inhibitors such as ripasudil and netarsudil for glaucoma therapy. Therefore, we aimed to evaluate the IOP lowering property of a new rho kinase inhibitor – SB77277B (SB77) under normal and cyclic mediated stress condition using HOCAS. We found that SB77 is capable of lowering IOP in a dose dependent manner as well as effective in alleviating the outflow resistance mediated by the cyclic mechanical stress. We are now at the process of developing SB77 as a clinical candidate for the management of glaucoma.
MicroRNA Role in Steroid-induced Ocular Hypertension
Steroid –induced ocular hypertension and glaucoma is an important clinical condition associated with the use of steroids in patients with various inflammatory or autoimmune diseases. Steroid -induced glaucoma is a secondary glaucoma associated with steroid use and is very common in subset of population (steroid responders). The responsiveness of steroid varies among the patients and the susceptible individuals are at great risk of developing optic neuropathy leading to blindness. It is reported that, 40% of the general population showed increased intraocular pressure (IOP) due to dexamethasone (steroid) use and 6% of these patients will develop glaucoma. More than 98% of the patients with POAG are steroid responders which eventually enhance the susceptibility of losing vision. However, the molecular basis for such responsiveness towards steroids is not very well understood.
Through India Alliance (IA-Intermediate Fellowship) funding programme and collaboration with Prof. Colin. E. Willoughby, Ulster University, Northern Ireland, UK , the research team is engaged to address why some patients under steroid (glucocorticoid) therapy for any inflammatory/ autoimmune diseases respond to steroids (steroid responders) and why some are more susceptible to develop secondary glaucoma and why not others?.
We believe that the microRNAs (small non-coding RNA) may have a regulatory role in mediating steroid receptor signaling and the development of miRNA mimics / inhibitors would be beneficial for Steroid-induced glaucoma. By proving this mechanism in an ex vivo model system using HOCAS, we would be able to predict the susceptible individuals with the specific set of microRNAs as a biomarker and to develop miRNA mimics / inhibitors for steroid glaucoma.
Drug Delivery Strategy for corneal infections
Our lab is also engaged in developing human amiotic membrane (HAM) as a drug reservoir. As a first step towards this goal, the research team has successfully evaluated drug impregnated HAM for its extended release of moxifloxacin as a model drug in vitro (Yelchuri et al., 2017). The same technique has been extended to evaluate other drugs such as anti-bacterials, and anti-fungals and anti-angiogenic agents.
Ongoing Projects
- Role of microRNA in regulating glucocorticoid receptor signaling in steroid-induced ocular hypertension/glaucoma
Funding Agency: India Alliance – Intermediate Fellowship (2016-2022)
Faculty
Clinician Scientist
Collaborators
- Paul Kaufman, University of Wisconsin, Madison, USA
- Colin. E. Willoughby, Ulster University, Northern Ireland, UK
Post-Doctoral Fellow
- Dr. Haribalganesh
Research Scholars
- Mr. K. Kathirvel
Lab Assistants:
- Ms. A.P. Bala
Human Organ Culture Anterior Segment (HOCAS) Facility for Trabecular Meshwork Outflow Studies
HOCAS facility has been established at AMRF in 2013 in collaboration with Prof. Paul L.Kaufman and B’Ann Gabelt, Department of Ophthalmology & visual sciences, University Wisconsin, Madison, USA. This facility was funded by Aravind Eye Foundation (AEF), New York, USA.
The human organ cultured anterior segment system (HOCAS) has been developed as an intermediate step between cell culture and whole animal or human studies for determining trabecular meshwork outflow facility responses to investigational drugs. This system allows direct experimentation of candidate drugs on human/bovine/pig and monkey eyes and thus provides direct and meaningful information about the candidate drugs for glaucoma. They are also useful for investigating the potential of gene therapy to target the trabecular outflow pathway.
The human anterior segment mounted onto a specially designed Petri dish is maintained at 37 ° C with 5% CO2 incubator (Thermo Fischer, USA) and continuously perfused with nutrient medium using PH D infusion only pump (Harvard Apparatus, USA). The other end of the eye is connected to APT 300 pressure transducers (Harvard Apparatus, USA) wand the pressure is monitored on a computer connected to the Powerlab system (8/35) (Quad Bridge Amplifier) with Lab Chart Pro software (Harvard Apparatus, USA).
HOCAS: Instrument Set-up
Services rendered:
- Hands-on training on Dissection, HOCAS set up, Drug treatment protocol and data analysis was provided to International student – Ms. Karen from Dr. Colin Willoughby’s Lab, University of Liverpool, UK as a part of research collaboration.
- established mechanical stress model to investigate the role of Rho A/ROCK signaling (SERB Project)
- Being used to establish steroid-induced ocular hypertension ex vivo model using HOCAS to understand the role of micro RNA in the pathogenesis of steroid-induced glaucoma as a part of research projects funded by the Wellcome Trust-DBT/India Alliance.
High Performance Liquid Chromatography (HPLC) for bio-analysis of Drugs

Equipment Setup
Shimadzu Prominence HPLC system with PDA detector (Shimadzu Corporation, Kyoto, Japan).
Services rendered:
- Investigated the ocular pharmacokinetics of voriconazole in aqueous humor of cataract patients (AMRF funded project)
- Estimated the levels of ascorbic acid in aqueous humor, lens and plasma of cataract patients to explore the genetic factors influencing ascorbic acid concentrations (AEH funded project)
- Estimated vitamin A in plasma of pateints with vernal conjunctivitis (AEH funded collaborative study)
- Estimated moxifloxacin, prednisolone, voriconazole, cefazolin and bevacizumab to investigate the human amniotic membrane (HAM) drug reservoir function (AEH funded project)
- Quantified plasma carotenoids (lutein, zeaxanthin, beta carotene and lycopene) as a part of IND-MACARE study funded by Indian Council of Medical Research
Tissue culture Facility
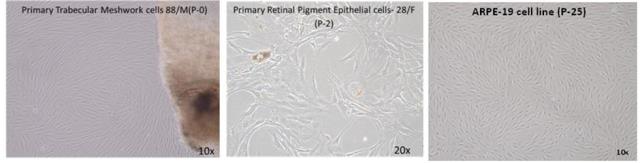
Our department maintains cell lines and primary cultures as follows:
- Tissue and cell culture- Maintenance of ARPE-19cell lines in monolayer.
- Primary culture (e.g. Retinal Pigment epithelial cells and Trabecular Meshwork cells from human donor eyes obtained from Rotary International Eye Bank of Aravind Eye Hospital, Madurai).
Services rendered:
- Evaluated the efficacy of Epalrestat, aldose reductase inhibitor in ARPE-19 cell line under hyperglycemia as a part of research project supported by DBT-Young Investigator Award.
- Evaluated some natural compounds –flavanoids for their aldose reductase inhibitory property using ARPE-19 cell line as a part of PhD thesis from our Indian collaborators
- Being utilized to establish primary HTM cells with known GC-responsiveness for mircoRNA manipulation experiments as a part of project objectives funded by the Wellcome Trust-DBT/India Alliance